What is polyethylene and why should we use it?
- Article
- What is polyethylene and why should we use it?

- July 4, 2023
- 9:22 am
What is polyethylene and why should we use it?
We would like to take a look at the definition of polymers before answering the question “what is polyethylene?”. Polymers are macromolecules and both synthetic and natural types of them are widely used in our daily lives. These substances are composed of monomers or similar and repeating subunits that are bonded to each other in chains.
 Polyethylene is a derivative of crude oil
Polyethylene is a derivative of crude oil
Now we can get back to the question “what is polyethylene?”. Polyethylene is a type of polymer consisted of ethylene as its subunit. Although crude oil is used to produce the raw material of polyethylene, other materials such as natural gas are also used in its production. In other words, the overall structure of this polymer is made from crude oil and natural gas.
It is important to know that this polymer is a granule; Therefore, it is produced by polymerization technique. You might ask if polyethylene can be considered a type of plastic? To answer this question, let us introduce different types of plastics to you. Plastics are generally classified into two categories of thermosetting and thermoplastic polymers.
The first group, thermosetting polymers are non-recyclable. Whereas, the second group, thermoplastic polymers are recyclable and used to manufacture tables, chairs, bottles, different containers, fuel tanks, insulators, etc. The polyethylene discussed in this article belongs to the second group.
This substance is used in pure form to produce different plastic compounds in industrial settings. Due to its nontoxic property polyethylene is widely used in different industries, especially in food industry.
History and discovery of polyethylene
 Discovery of polyethylene
Discovery of polyethylene
In order to learn more about a substance and find an answer to the question “what is polyethylene?” it is important to pay attention to the history, naming, and applications of that substance in the past. As you know, most of the widely-used materials in history were discovered by accident. This kind of accidental discoveries mostly happened as a result of experimenting with several substances to get a different result. Interestingly, materials produced through such methods would later have wide applications.
To find an answer to the question “what is polyethylene?” it is necessary to note that this substance was first synthesized by total accident. It happened when a German chemist heated Diazomethane for the first time in 1898 and the result was a white waxy compound. It is worth to know that the first industrial synthesis of this substance which would later be named polyethylene was also discovered by total accident.
In other words, five years after the first synthesis of this compound in 1933 two chemists, Eric Fawcett and Reginald Gibson heated Benzaldehyde and Ethylene under high pressure and successfully produced the waxy compound called polyethylene. The reaction had happened because the oxygen leaking inside the reaction chamber had acted as the initiator of polymerization.
This led to polyethylene reproduction by Michael Perrin two years later in 1935. It is interesting to know that the method Michael Perrin used to synthesize Polyethylene under high pressure later (in 1939) became a fundamental method in industrial production of a substance called LDPE.
The structure of this incredible polymer
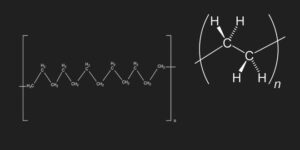 The structure of polyethylene
The structure of polyethylene
Now that you have learned the history of polyethylene production we would like to explain the structure of this substance using the information above. As we know, polyethylene which is known as a waxy and inactive solid is produced by polymerization of ethylene.
The double bond in the ethylene molecule breaks during polymerization. After the bond breaks, a simple bond forms between carbon atoms in monomers and leads to the formation of polyethylene macromolecule.
Different materials might be produced only with small modifications in the molecular structure of this polymer. This might be possible with the crystallization and modifications in chain positions. Another way to produce different materials with this polymer is to change the length of the chains by changing the molecular weight of the polymer. In addition to that, a change in the chemical bond between the chains will lead to a change in the properties of the branches and help create a different material.
Properties of Polyethylene
As mentioned in the section “what is polyethylene?” it is possible to produce different materials by creating changes in the structure of the polymer. However, you need to note that all types of polyethylene have similar chemical characteristics such as:
- Polyethylene cannot absorb water and has a high chemical stability.
- This polymer is highly permeable by carbon dioxide and oxygen. Whereas, water vapor and polar gases can hardly permeate it.
- These polymers burn with a blue flame and give off paraffin odor when burned.
- They break down in sunlight. That is why carbon black might be used to make them UV resistant.
- They have similar chemical characteristics with paraffin.
Major types of polyethylene
 Polyethylene
Polyethylene
In the next section of “what is polyethylene?” we will introduce different types of these polymers. It is necessary to know that polyethylene has the highest rate of production and application among all polymers. We might generally explore different types of this polymer from two aspects. But first we need to introduce the major types of polyethylene:
- LDPE or low density polyethylene: it has a high flexibility and is produced through free-radical polymerization. In addition to this, it has a low intermolecular force and tensile strength and degradable by microorganisms.
- HDPE or high density polyethylene: this type of polymer is produced through Ziegler-Natt catalyst polymerization. It has a high intermolecular force and tensile strength and lacks branches in its chains.
- LLDPE or linear low density polyethylene
Classification of polyethylene based on density
 Polyethylene
Polyethylene
In this section of “what is polyethylene?” we will introduce a new classification of polyethylene based on density:
- HDPE or high density polyethylene
- MDPE or medium density polyethylene
- LDPE or low density polyethylene
- LLDPE or linear low density polyethylene
- VLDPE or very low density polyethylene
- PEX or XLPE or cross-linked polyethylene
- UHMWPE or ultra-high molecular weight polyethylene
Applications and advantages of polyethylene
 What are different applications of polyethylene?
What are different applications of polyethylene?
At the final part of “what is polyethylene?” we need to remind you that these polymers have a wide daily and commercial applications. Some of these applications are as follows:
- In most cases, HDPE is used to manufacture all types of polyethylene tanks, different polyethylene pipes and fittings. This type of polyethylene is also used to produce shipping boxes, plastic items, packaging bags, etc.
- LDPE is used to manufacture polymer sheets, plastic bags, packaging films, etc.
- MDPE is used to produce protective coating for metal surfaces and all types of pipes and fittings for pressurized irrigation.
- LLDPE is used to produce covering for telecommunication lines including power cables, all plastic components and coatings.
- Polyethylene with higher molecular weight is used to manufacture special clothes, bulletproof vests, and certain components of industrial machinery.
- Polyethylene is also used to produce the main auto parts, home appliances, toys, plastic equipment used in medicine, etc.




